Iron – although present in your body in an amount of only about 4 grams—plays a crucial role, participating in over 180 biochemical reactions! As an integral component of hemoglobin, it is essential for transporting oxygen from the lungs to every cell in the body (except for red blood cells themselves, which rely on anaerobic metabolism). Iron is also a key component of myoglobin, the oxygen-storing protein in muscles. Additionally, this element plays a vital role in protecting cells from oxidative damage, supports the production of hormones responsible for neurotransmission, and is involved in the formation of DNA, RNA, and collagen. Iron also strengthens our immune system by supporting the production and function of white blood cells. In what forms does iron appear in the diet, and what influences its absorption? Which foods are rich in iron? What is the optimal iron level in the blood? How can possible iron deficiencies be recognized, and what are the effects of its excess? You will find the answers to these questions in the following section of the article!
Authors: M.Sc. Eng. Klaudia Buczek, M.Sc. Pharm. Michał Miśta
Table of Contents:
- Types and Bioavailability of Iron
- Best Sources of Iron
- Daily Iron Requirements
- Iron Metabolism in the Body
- Testing and Prevention
- Symptoms of Iron Deficiency
- Iron Deficiency Without Anemia
- Anemia (Iron Deficiency Anemia)
- Iron Deficiency in Children
- Iron Deficiency in Adolescents
- Iron in Pregnancy – Risks and Consequences of Deficiency
- Iron Deficiency and Overweight/Obesity
- Iron Deficiency in Athletes
- Iron Deficiency and Vegetarian Diets
- Iron Deficiency and Vegan Diets
- Iron Deficiency in the Elderly
- What Hinders Iron Absorption (“Leaches Iron”)?
- Iron Absorption Enhancers
- Iron Supplementation
- Symptoms of Iron Excess: Hemochromatosis
- Summary
- References
1. Types and Bioavailability of Iron
Iron found in food exists in two forms, which differ in their degree of absorption:
- Heme Iron – This is iron in its reduced state (Fe²⁺). Heme iron is combined with a heme molecule and is primarily found in animal products such as meat and offal. It is absorbed with an efficiency of about 20-25%, meaning the body can absorb up to a quarter of the iron present in meat, which then enters the bloodstream. A distinctive feature of heme iron is that its absorption is not significantly affected by other dietary components.
- Non-Heme Iron – This is iron in its oxidized state (Fe³⁺), found mainly in plant-based foods such as wheat bran, cocoa, pumpkin seeds, sesame seeds, and legumes. The absorption of this form is much lower, at only 2-5%. Moreover, the absorption of non-heme iron can be significantly reduced by other dietary components, such as oxalates and phytic acid, which can
Heme iron, derived from meat, accounts for only 10% of the total iron in the diet, while the non-heme form, originating from plant sources, makes up as much as 90%. As a result, in a balanced diet and in the absence of absorption disorders, the average absorption rate of iron is about 10% of the consumed amount. This rate increases 2–3 times in cases of iron deficiency in the body [36].
Interestingly, the iron found in breast milk has the highest absorption rate among all foods, reaching around 50%. This is primarily due to iron bound to lactoferrin, a protein that chelates iron ions, aiding their transport and absorption in the intestines of infants.
2. Best Sources of Iron
Now that you know which forms of iron are best absorbed from the gastrointestinal tract, you may be wondering what the best sources of this element are in your daily diet. Let’s take a closer look at them.
Animal Sources of Iron
Heme iron is mainly found in organ meats such as liver, kidneys, and heart. These organs are particularly high in iron because they play crucial metabolic roles related to the storage and transport of this mineral. Red meat, especially beef and lamb, and meat products containing blood, such as black pudding, also have a high iron content.
Fish, especially those with darker meat such as sardines, herring, tuna, cod, and salmon, also contain heme iron due to the presence of hemoglobin in the blood and myoglobin in muscle tissues. However, like poultry, fish have relatively lower iron content compared to red meat.
Mussels, like other mollusks (e.g., oysters and clams), mainly contain non-heme iron because they use hemocyanin as their primary respiratory pigment instead of hemoglobin. Although non-heme iron from mussels is less absorbable than heme iron, it is still better absorbed than plant-based iron due to the presence of amino acids, such as histidine and other iron absorption enhancers.
Figure 1. Best Sources of Heme and Non-Heme Iron. Pork liver, chicken liver, and red meat are rich in heme iron, while plant-based sources of iron, such as wheat bran, oats, cocoa, pumpkin seeds, nuts, broccoli, and peas, provide non-heme iron. Although chicken eggs are an animal product, they contain non-heme iron. Contrary to popular belief, apples, tomatoes, and avocados contain minimal amounts of iron, but their relatively high vitamin C content can support the absorption of non-heme iron from other dietary sources.
Plant-Based Sources of Iron
Non-heme iron, found in plant-based foods, is present in a wide variety of products. Rice bran, available in health food stores, is particularly high in iron and is one of the best plant-based sources of this mineral. Significant amounts of iron are also found in wheat bran, whole grains, whole wheat flour, whole grain bread, coarse groats, and wheat germ. Legumes, such as lentils, beans, and chickpeas, also contain substantial amounts of iron. Oats, nuts, pumpkin seeds, and sesame seeds—among the richest plant-based sources of iron—provide this micronutrient in large quantities. Cocoa is also an excellent source of iron, and regular consumption can help replenish deficiencies while providing additional valuable nutrients.
Iron content in green vegetables such as spinach, parsley (leaves and root), chives, sorrel, as well as chard, beets, and Brussels sprouts is also quite significant, although its absorption may be limited by the presence of oxalates. Yellow bell pepper, although less commonly associated with iron, is also a source of this mineral and aids in its absorption due to its vitamin C content. Surprisingly, egg yolk (an animal product) is also high in non-heme iron!
In the iron-rich foods table below, you will find a detailed breakdown of iron content in both plant-based and animal-based products (per 100 g).
| Iron-Rich Foods | Iron Content per 100 g | Type of Iron |
|---|---|---|
| Iron-Rich Meat Products | ||
| Pork liver | 18,7 mg | Heme (absorbable at 20-25%) |
| Chicken liver | 9,5 mg | |
| Beef liver | 9,4 mg | |
| Veal liver | 7,9 mg | |
| Chicken heart | 6,0 mg | |
| Chicken kidneys | 5,2 mg | |
| Pork kidneys | 4,9 mg | |
| Beef kidneys | 4,6 mg | |
| Blood sausage (varies by ingredients and preparation method) | 4 – 7 mg | |
| Hunter’s sausage | 2,5 mg | |
| Veal (leg) | 2,4 mg | |
| Goose | 2,4 mg | |
| Beef (roast) | 2,3 mg | |
| Duck | 2,1 mg | |
| Krakowska sausage, dry | 1,5 mg | |
| Turkey (breast) | 0,5 mg | |
| Chicken (breast) | 0,4 mg | |
| Iron-Rich Fish and Seafood | ||
| Clams | 23,8 mg | Non-Heme (absorbable at 2-5%) |
| Oysters | 7,8 mg | |
| Octopus | 5,3 mg | |
| Mussels (varies by type and origin) | 4 – 6 mg | |
| Caviar | 11,9 mg | Heme (absorbable at 20-25%) |
| Sardines (fresh or canned) | 2,9 mg | |
| Smoked mackerel | 1,5 mg | |
| Salted herring | 1,1 mg | |
| Tuna (fresh) | 1,0 mg | |
| Smoked cod | 1,0 mg | |
| Salmon (fresh, varies by type and source) | 0,3 – 0,6 mg | |
| Pollock (fresh) | 0,3 mg | |
| Sole | 0,2 mg | |
| Hake | 0,2 mg | |
| Pangasius | 0,2 mg | |
| Haddock | 0,1 mg | |
| Whole chicken eggs | 2,2 mg | Non-Heme (absorbable at 2-5%) |
| Iron-Rich Dairy Products | ||
| Edam cheese | 0,6 mg | Non-Heme (absorbable at 2-5%) |
| Emmental cheese | 0,5 mg | |
| Low-fat cottage cheese | 0,2 mg | |
| Full-fat cottage cheese | 0,2 mg | |
| Whole milk (3.2% fat) | 0,1 mg | |
| Milk (2% fat) | 0,1 mg | |
| Natural yogurt (2% fat) | 0,1 mg | |
| Iron-Rich Grain Products | ||
| Rice bran | 20 mg | Non-Heme (absorbable at 2-5%) |
| Wheat bran | 14,9 mg | |
| Wheat germ | 9,0 mg | |
| Quinoa | 8,9 mg | |
| Amaranth | 7,8 mg | |
| Millet | 4,8 mg | |
| Oatmeal | 4,25 mg | |
| Buckwheat | 2,8 mg | |
| Whole rye bread | 2,3 mg | |
| Graham bread | 2,2 mg | |
| Pearl barley | 1,6 mg | |
| Egg pasta | 1,4 mg | |
| Wheat flour (Type 500) | 1,1 mg | |
| Semolina | 0,9 mg | |
| Iron-Rich Legumes | ||
| Soybeans (dry seeds) | 8,9 mg | Non-Heme (absorbable at 2-5%) |
| White beans (dry seeds) | 6,9 mg | |
| Red lentils (dry seeds) | 5,8 mg | |
| Peas (dry seeds) | 4,7 mg | |
| Iron-Rich Vegetables | ||
| Dill | 6,6 mg | Non-Heme (absorbable at 2-5%) |
| Parsley (leaves) | 5,3 – 6,2 mg | |
| Peas | 4,7 mg | |
| Potatoes | 3,2 mg | |
| Spinach | 2,8 mg | |
| Swiss chard | 2,2 mg | |
| Beets | 1,7 mg | |
| Kale | 1,7 mg | |
| Canned tomatoes | 1,3 mg | |
| Parsley (root) | 1,1 mg | |
| Brussels sprouts | 0,9 mg | |
| Broccoli | 0,9 mg | |
| Corn (cob) | 0,8 mg | |
| Lettuce | 0,7 mg | |
| Tomatoes (fresh) | 0,5 mg | |
| Cherry tomatoes (fresh) | 0,24 mg | |
| Iron-Rich Fruits | ||
| Goji berries | 6,8 mg | Non-Heme (absorbable at 2-5%) |
| Dried apricots | 2,7 – 3,6 mg | |
| Dried figs | 2,0 – 3,3 mg | |
| Raisins | 2,3 mg | |
| Lychee | 1,7 mg | |
| Pitted prunes | 1,5 mg | |
| Black currants | 1,2 mg | |
| Raspberries | 0,8 mg | |
| Blueberries | 0,7 mg | |
| Avocado | 0,6 mg | |
| Apples | 0,3 mg | |
| Iron-Rich Nuts, Seeds, and Kernels | ||
| Sesame seeds | 14,6 mg | Non-Heme (absorbable at 2-5%) |
| Pumpkin seeds | 10,0 mg | |
| Blue poppy seeds | 9,8 mg | |
| Chia seeds | 7,7 mg | |
| Cashews | 6,7 mg | |
| Flaxseeds | 5,7 mg | |
| Pine nuts | 5,4 mg | |
| Hazelnuts | 4,7 mg | |
| Pistachios | 3,9 mg | |
| Almonds | 3,7 mg | |
| Sunflower seeds | 2,8 mg | |
| Brazil nuts | 2,5 mg | |
| Pecans | 2,4 mg | |
| Macadamia nuts | 1,9 mg | |
| Walnuts | 1,8 mg | |
| Other Iron-Rich Products | ||
| 99% Dark chocolate | 13,9 mg | Non-Heme (absorbable at 2-5%) |
| 70 – 85% Dark chocolate | 11,9 mg | |
| Cocoa 16%, powder | 10,7 mg | |
| Dried porcini mushrooms (porcini) | 8,4 mg | |
| 60 – 69% Dark chocolate | 6,3 mg | |
| 45-59% Dark chocolate | 8,0 mg | |
| Baker’s yeast | 5,0 mg | |
| Tofu | 2,8 mg | |
| Milk chocolate | 2,4 mg | |
Table 1. Iron-Rich Foods. Iron in natural food products exists in heme and non-heme forms, which differ in bioavailability. Animal products, such as meat and organ meats, are primarily sources of heme iron, which is more easily absorbed by the body. Plant-based foods and some seafood contain non-heme iron, which is less easily absorbed, but its uptake can be enhanced by dietary components like vitamin C. If you are wondering what to eat when facing iron deficiency, it is worth paying attention to various iron-rich foods. For ease of use, the products in the table above are divided into categories and organized in descending order of iron content, with information on whether they contain heme or non-heme iron.
3. Daily Iron Requirements
Iron requirements vary depending on gender, age, and health status. Healthy adult men need about 1 mg of absorbed iron per day, primarily due to the shedding of intestinal epithelial cells and other natural physiological processes, which is relatively easy to achieve. However, adult women of reproductive age need about 2 mg of absorbed iron daily due to monthly blood loss during menstruation. Since the average iron absorption rate is around 10%, men should consume about 10 mg of iron per day, while menstruating women need up to 18 mg to provide their bodies with the appropriate amount of this mineral. This can be challenging but achievable with a well-balanced diet!
Iron Requirements – RDA Standards
The recommended daily iron intake [12] for adults is:
- Women (19-50 years) – 18 mg
- Women (>50 years) – 10 mg
- Men (>19 years) – 10 mg
For other groups:
- Infants (0-6 months) – 0.3 mg
- Infants (7-11 months) – 11 mg
- Children (1-3 years) – 7 mg
- Children (4-9 years) – 10 mg
- Girls (10-12 years, before menstruation) – 10 mg
- Girls (10-12 years, after menstruation begins) – 15 mg
- Boys (10-12 years) – 10 mg
- Girls (13-18 years) – 15 mg
- Boys (13-18 years) – 12 mg
- Pregnant women – 27 mg
- Breastfeeding women – 10 mg
During pregnancy and periods of significant blood loss, the body’s iron requirement increases to about 3 mg per day, meaning that iron intake should be raised to 27 mg per day.
The above values refer to the total iron content in consumed food, which includes both heme and non-heme sources of this element.
Is consuming only the foods richest in iron the best approach? Not necessarily. Organ meats, although rich in iron, are not typically eaten every day. Excessive consumption of red meat, especially regular intake of processed products such as sausages or blood sausage, may be detrimental to health. These products, due to the presence of saturated fatty acids and carcinogenic compounds formed during heat processing, such as nitrosamines and heterocyclic amines, can increase the risk of cardiovascular diseases and cancer.
The same applies to dark chocolate. Although it contains significant amounts of iron, it is non-heme iron, which is less absorbable. Additionally, chocolate contains some tannins, which can limit the absorption of this mineral. It’s also important to remember that due to its high content of saturated fats and often added sugar, a single bar can provide over 600 kcal!
It’s worth noting that while products like cocoa and parsley leaves are rich in iron, it is difficult to consume them in quantities that would provide a significant amount of this mineral. A valuable exception is wheat and rice bran, which can be an extremely beneficial addition to the diet, especially for those seeking plant-based sources of iron, while also providing fiber, B vitamins, and minerals such as magnesium and zinc. However, keep in mind that the iron found in bran is also non-heme iron. Therefore, it is recommended to consume these foods alongside those that enhance iron absorption, such as vitamin C, as discussed in Chapter 9.
How, then, can you best meet your daily iron requirements? The optimal strategy is to combine both plant-based and animal-based sources of iron in your diet, taking into account the typical portion sizes of each product. You can achieve this even on a low-calorie diet!
Sample Daily Menu for Women
If you’re a woman following a 1600 kcal diet, your daily meal plan can consist of 3 simple meals that together provide over 20 mg of iron! How can you achieve this?
- For breakfast, prepare oatmeal with 2 tablespoons of pumpkin seeds, a tablespoon of cocoa, and a handful of blueberries.
- For lunch, enjoy tomato spaghetti with beef.
- For dinner, opt for a Greek salad with added lentils.
You can find a detailed list of all the products, along with their iron content, in Table 2.
| Product | Serving Size | Iron Content per 100 g | Iron Content per Serving | Type of Iron |
|---|---|---|---|---|
| Breakfast – Oatmeal with Additions | ||||
| Oats | 6 tablespoons (60 g) | 4,25 mg | 2,55 mg | Non-Heme |
| Milk* 2% | 1 cup (250 g) | 0,10 mg | 0,25 mg | Non-Heme |
| Pumpkin seeds | 2 tablespoons (20 g) | 10,0 mg | 2,0 mg | Non-Heme |
| Cocoa powder | 1 tablespoon (10 g) | 13,0 mg | 1,39 mg | Non-Heme |
| Blueberries | 1 handful (50 g) | 0,28 mg | 0,14 mg | Non-Heme |
| Lunch – Spaghetti Bolognese | ||||
| Olive oil | 1 tablespoon (10 g) | 0,60 mg | 0,06 mg | Non-Heme |
| Ground beef | 1 serving (100 g) | 1,99 mg | 1,99 mg | Heme |
| Canned diced tomatoes |
0,5 can (200 g) | 1,30 mg | 2,60 mg | Non-Heme |
| Whole wheat pasta | 1 cup (90 g) | 3,62 mg | 3,26 mg | Non-Heme |
| Red bell pepper | 1 small piece(75 g) | 0,60 mg | 0,45 mg | Non-Heme |
| Dinner – Salad with Feta Cheese | ||||
| Arugula | 2 handfuls (40 g) | 1,45 mg | 0,58 mg | Non-Heme |
| Cherry tomatoes | 1 handful (100 g) | 0,24 mg | 0,24 mg | Non-Heme |
| Feta cheese | 2,5 slices (50 g) | 0,66 mg | 0,33 mg | Non-Heme |
| Marinated green olives | 1 tablespoon (15 g) | 0,46 mg | 0,07 mg | Non-Heme |
| Red lentils | 3 tablespoons (60 g) | 7,38 mg | 4,43 mg | Non-Heme |
| Total Iron Intake for the Day: | 20,34 mg | Non-Heme (18,35 mg) Heme (1,99 mg) |
||
Table 2. Sample Daily Menu for a Woman on a Low-Calorie Diet (1600 kcal), Demonstrating the Possibility of Ensuring Adequate Iron Intake.
*If you wish to further enhance iron absorption, choose plant-based milk, such as unsweetened soy milk not fortified with calcium, and drink your meals with water mixed with freshly squeezed lemon juice.
Diversifying iron sources allows for better meeting of the body’s iron requirements, helping to maintain health and a balanced diet.
4. Iron Metabolism in the Body
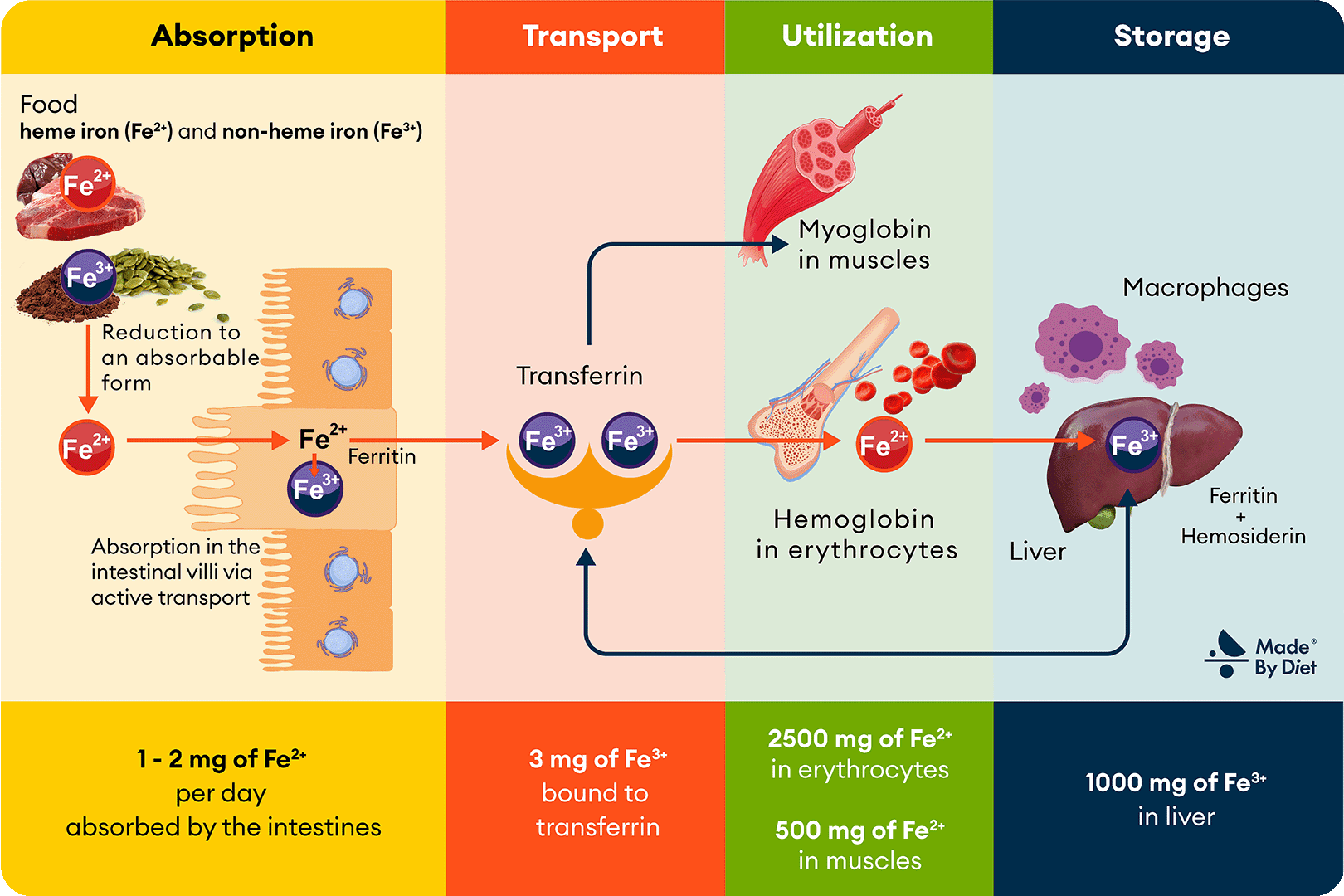
Let’s now take a closer look at the transformations of iron that occur in the human body, which are crucial for understanding issues such as deficiencies. Although these processes may seem complex, the following Image 2, divided into 4 sections according to the subpoints in the text, will help you visualize them.
Figure 2. Iron Metabolism. Iron ingested with food is absorbed in the intestines and then transported by transferrin to tissues, where it participates in the formation of hemoglobin, myoglobin, and other metalloproteins (including cytochromes, catalases, and peroxidases). Excess iron is stored in the liver as ferritin and hemosiderin, and iron from the breakdown of erythrocytes is recycled with the involvement of macrophages and bone marrow.
Iron Absorption
Iron absorption is a crucial stage in its metabolism, determining how much of this mineral is available to the body. This process primarily takes place in the small intestine and varies depending on the type of iron present in the diet (heme iron from meat or non-heme iron from plant sources).
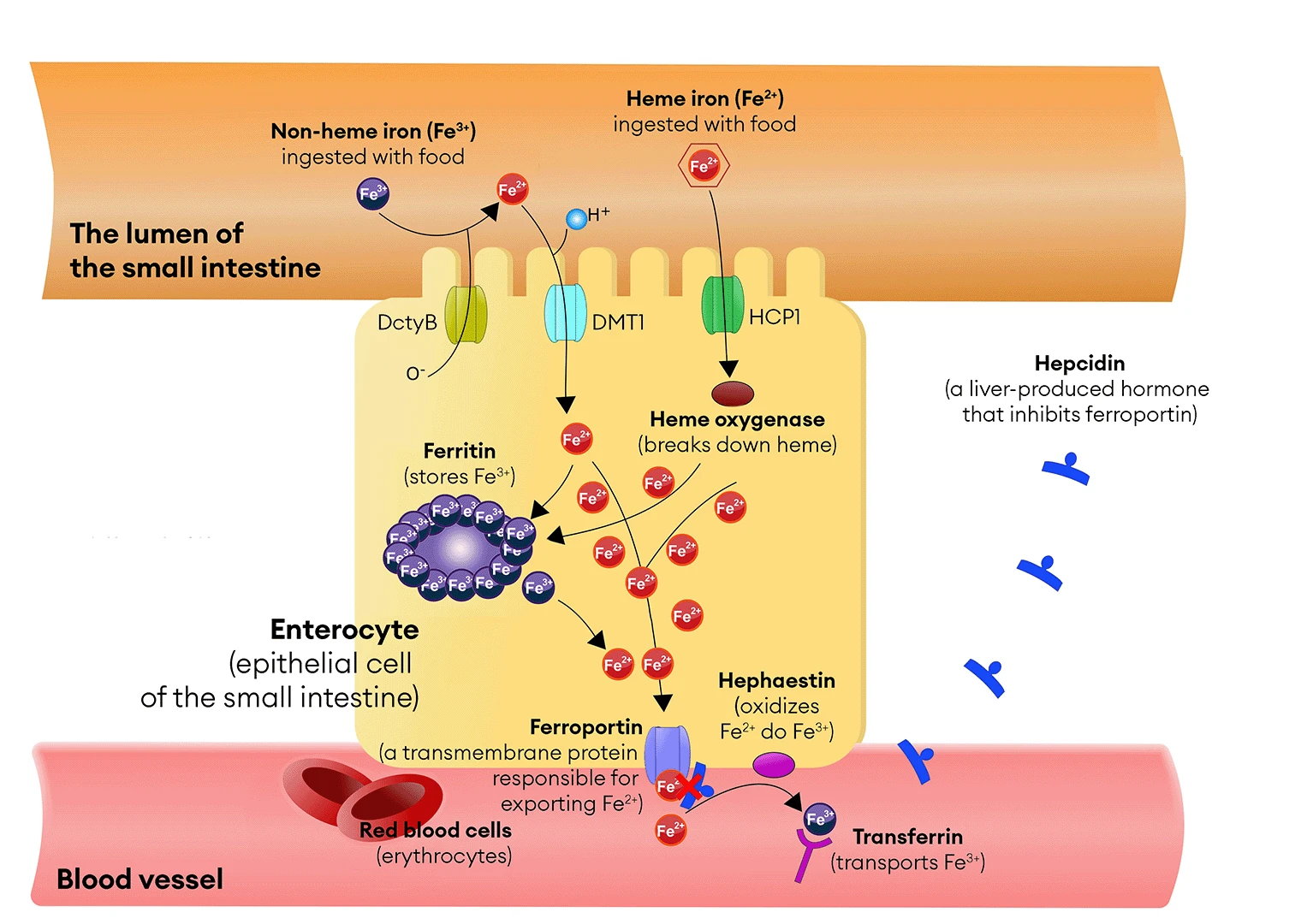
Absorption of Heme Iron
During the digestion of meat, proteins such as hemoglobin and myoglobin break down, releasing heme—a non-protein component containing iron in its ferrous state (Fe²⁺). Heme is absorbed in the small intestine, primarily in the duodenum, in its unchanged form. The absorption of heme occurs in specialized intestinal cells equipped with microvilli, which increase their absorptive surface area. These cells, known as enterocytes, have specific receptors on their surface called heme transporters (e.g., Heme Carrier Protein 1 – HCP1), which bind heme and facilitate its transport into the cell (see Image 3). In the cytoplasm of enterocytes, heme is broken down by the enzyme heme oxygenase, releasing iron ions (Fe²⁺).
Absorption of Non-Heme Iron
Plant-based foods contain non-heme iron, which primarily exists as ferric iron ions (Fe³⁺). In the stomach, this iron is partially released from the food and then reduced to ferrous ions (Fe²⁺) on the surface of enterocytes by the enzyme duodenal cytochrome B reductase (DcytB – Duodenal Cytochrome B). Fe²⁺ ions are then absorbed by the DMT1 transporter (Divalent Metal Transporter 1). As mentioned in Chapter 2, this process is less efficient than heme iron absorption and depends on other dietary components. Vitamin C can enhance non-heme iron absorption, while compounds such as phytates and polyphenols can inhibit it (see Chapters 8 and 9).
Iron Transport – The Role of Transferrin
After iron is absorbed in the intestines, Fe²⁺ ions pass through the enterocyte membranes with the help of specialized proteins that act as transporters—DMT1 (Divalent Metal Transporter 1), which carries the ions into the cell, and ferroportin, which allows them to enter the bloodstream. In the blood, Fe²⁺ is oxidized to Fe³⁺ by hephaestin, a copper-containing transmembrane oxidase that functions similarly to ceruloplasmin, the main copper carrier in the body. The resulting ferric iron ions (Fe³⁺) can then be effectively bound by transferrin, a protein responsible for iron transport in the blood. Transferrin binds these ions selectively and precisely, ensuring their safe transport and minimizing the risk of toxic effects associated with excessive iron concentrations in the bloodstream.
Figure 3. Iron absorption occurs in the epithelial cells of the small intestine, known as enterocytes, and is regulated by the hormone hepcidin, produced by the liver. Hepcidin is a key hormone regulating iron homeostasis in the body. The abbreviations used in the illustration are: DcytB (Duodenal Cytochrome) – duodenal reductase, DMT1 (Divalent Metal Transporter 1) – Fe²⁺ ion transporter, BHCP1 (Heme Carrier Protein 1) – heme transporter.
Iron Utilization – Erythropoiesis and the Role of Bone Marrow
Bone marrow cells capture the complex of ferric iron ions and transferrin through transferrin receptors (transferrin receptor protein 1, TfR1), whose synthesis increases during periods of heightened demand for red blood cells (erythrocytes), such as during regular high-intensity endurance training. In the bone marrow, iron is used for the biosynthesis of heme, which is then incorporated into hemoglobin molecules—the key protein found in red blood cells that is responsible for oxygen transport (see Image 4). The process of red blood cell production in the bone marrow is known as erythropoiesis.
Iron is also an integral part of myoglobin, a muscle protein that stores oxygen in muscles, allowing for its rapid release during intense physical activity. Additionally, iron is involved in the biosynthesis of numerous enzymes, such as cytochromes, catalases, and peroxidases.
Iron Storage – The Role of Ferritin and Hemosiderin
An excess of iron can be harmful to the body, leading to the formation of oxygen free radicals (reactive oxygen species), which can damage cells and tissues and contribute to the development of conditions such as hemochromatosis (see Chapter 11). To avoid this risk, the body has developed a precise mechanism for managing iron stores, allowing for effective storage and release while minimizing the risk of toxicity.
Iron in the body is stored in tissue reservoirs such as the liver, spleen, bone marrow, and the aforementioned enterocytes, primarily in the form of the storage protein ferritin. When the body needs iron, ferritin releases it, and Fe³⁺ is reduced back to Fe²⁺ and transported into the bloodstream. In cases of iron excess, it remains in its bound form until it is utilized or excreted, protecting the body from the harmful effects of excessive iron levels in the bloodstream. Thus, ferritin acts as a buffer, storing iron in a safe form and releasing it when needed by the body.
When iron stores are high or its absorption is excessive, some iron is stored in the form of hemosiderin. Hemosiderin is formed from ferritin due to prolonged or excessive iron accumulation and is stored in macrophages and the liver. Unlike ferritin, iron from hemosiderin is less readily available to the body, meaning it cannot be used as easily or quickly. However, this property allows hemosiderin to help protect the body from potentially toxic levels of free iron, providing long-term storage of the mineral.
Iron Recycling – Role of Macrophages
Macrophages, as part of the immune system, play a crucial role in managing iron resources. These cells phagocytize old and damaged erythrocytes, breaking them down and releasing the iron contained in hemoglobin, which can be reused by the body. This process is highly efficient—about 95% of the iron needed for the synthesis of new erythrocytes comes from the recycling of iron from used red blood cells. Each day, the body processes around 20 mg of iron, primarily through the recycling of destroyed erythrocytes, illustrating how dynamic this process is.
Iron Regulation – Role of Hepcidin
The body has a precise mechanism for regulating iron levels to maintain homeostasis and prevent both deficiencies and the toxic effects of excess iron. A key player in this process is the hormone hepcidin, which is primarily produced in the liver.
Hepcidin works by inhibiting ferroportin, a transmembrane protein responsible for exporting iron from cells into the bloodstream. Ferroportin is found not only in enterocytes (intestinal cells) but also in macrophages, hepatocytes (liver cells), and spleen cells.
In cases of iron excess, elevated hepcidin levels inhibit iron absorption and release, reducing its availability in the bloodstream. This helps maintain balance and ensure appropriate iron levels in the body. Similarly, hepcidin levels can rise in response to inflammation, infections, or intense physical exercise, leading to reduced iron availability. The increase in hepcidin levels following physical exercise is a protective mechanism that shields the body from excessive oxidative stress and the potentially harmful effects of free iron in the bloodstream.
Conversely, in situations of iron deficiency or increased demand for this mineral (e.g., during pregnancy or periods of rapid growth), hepcidin levels decrease, enhancing iron absorption and release into the bloodstream.
Hepcidin is also subject to daily regulation—its levels naturally rise in the morning and decrease in the evening. This is important in the context of iron supplementation, as taking iron in the evening, when hepcidin levels are lower, may improve the efficiency of iron absorption.
The regulation of iron levels by hepcidin is crucial for maintaining the proper balance of this mineral, which is essential for human health. Disruptions in the production or function of hepcidin can lead to serious health problems, such as anemia or hemochromatosis.
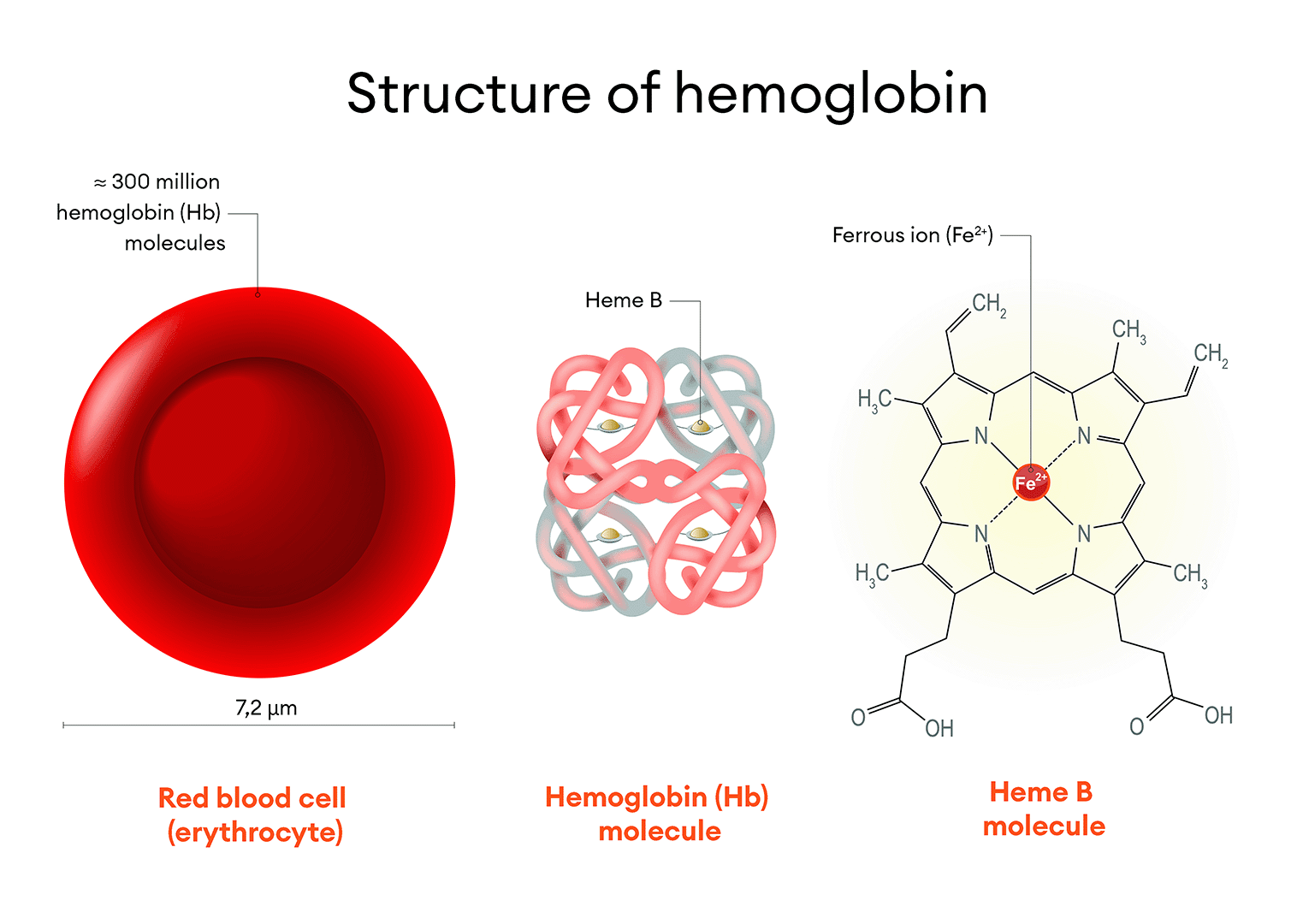
Figure 4. Structure of the Hemoglobin Molecule. Red blood cells (erythrocytes) are the most abundant cell population in the blood. Each erythrocyte contains approximately 300 million hemoglobin molecules. Each hemoglobin molecule has four heme groups, with a central ferrous iron ion. Erythrocytes are the primary carriers of iron in the body, storing about two-thirds of the total amount of this mineral.
5. Testing and Prevention
Regular monitoring of iron levels is an important aspect of a healthy lifestyle, especially if you belong to a high-risk group, such as adolescents during puberty, women with heavy menstrual periods, pregnant women, athletes, individuals with chronic bleeding (e.g., from the gastrointestinal tract or reproductive organs), and patients after major surgeries.
Iron in the Blood – Which Tests to Perform?
Here are the key tests useful to diagnose possible iron deficiency:
- Blood Count (CBC) – A basic test that evaluates:
- Red Blood Cell Count (RBC): Assesses the blood’s oxygen-carrying capacity.
- Hematocrit (HCT): The percentage of red blood cells in the blood volume.
- Hemoglobin Concentration (HGB): Indicates the level of hemoglobin in the blood, which may suggest iron deficiency.
- Mean Corpuscular Volume (MCV): Assesses the average size of red blood cells.
- Mean Corpuscular Hemoglobin (MCH): Measures the amount of hemoglobin per red blood cell.
- Mean Corpuscular Hemoglobin Concentration (MCHC): Evaluates the saturation of red blood cells with hemoglobin.
- Red Cell Distribution Width (RDW): Determines the variation in the size of red blood cells.
- Biochemical Tests – Evaluate iron metabolism:
- Serum Iron Level: Measures the amount of free (unbound to transferrin) iron in the blood.
- Total Iron-Binding Capacity (TIBC): Determines the amount of free transferrin available to bind iron.
- Ferritin Level: A protein that stores iron, indicating the body’s iron reserves.
- Transferrin Level: The iron-transporting protein, helpful in assessing the efficiency of iron transportation.
Regularly conducting these tests and having them properly interpreted by an experienced clinical dietitian or physician allows for the early detection of issues related to low iron levels and enables effective intervention.
What Are the Recommended Iron Levels?
Reference ranges for total iron concentration in serum may vary depending on the laboratory. The standard values are:
- Infants up to 6 months: 35–155 µg/dl; 6.27–27.75 μmol/L
- Children from 6 months to 15 years: 45–185 µg/dl; 8.06–33.12 μmol/L
- Girls over 15 years: 40–145 µg/dl; 7.16–25.96 μmol/L
- Boys over 15 years: 55–160 µg/dl; 9.84–28.64 μmol/L
- Women: 55–180 µg/dl; 9.84–32.22 μmol/L
- Men: 70–200 µg/dl; 12.53–35.8 μmol/L
Blood test results should always be interpreted according to the reference ranges provided by the test provider. An iron level above the normal range is marked with the symbol ↑ or H (high), while a level below the normal range is marked with the symbol ↓ or L (low).
Remember that iron levels in the blood can fluctuate throughout the day (e.g., they may be 30% higher in the morning) and do not always reflect the body’s actual needs. Therefore, they are not an ideal indicator of health status. A better marker of iron deficiency anemia is a complete blood count (CBC) test. A characteristic feature of this type of anemia is a reduction in the average size of red blood cells (MCV). A decreased MCV, along with lower hemoglobin, hematocrit, and red blood cell count, is known as microcytic anemia, typical of iron deficiency, which is often treated through diet (diet therapy).
Figure 5. Iron Deficiency and Health. Regular monitoring of iron levels is a crucial aspect of a healthy lifestyle, especially for those at risk of iron deficiency, such as adolescents during puberty, women with heavy menstrual bleeding, pregnant women, athletes, individuals with chronic bleeding (e.g., from the gastrointestinal tract or reproductive organs), and patients who have undergone major surgeries. Iron deficiency in adults can lead to serious health issues, making regular checks of iron levels essential.
6. Symptoms of Iron Deficiency
Every day, the body loses small amounts of iron, approximately 1 mg, primarily through the shedding of intestinal epithelial cells (as discussed in Chapter 4). Although this amount may seem minor, it must be replenished regularly to prevent deficiencies. In menstruating women, additional iron loss occurs due to monthly blood loss, significantly increasing their daily iron requirements. Individuals at risk of iron deficiency include those with increased needs, undergoing rapid growth, limiting iron sources in their diet, or facing absorption disorders. These groups include pregnant women, children and adolescents in rapid growth phases, athletes, vegetarians, and the elderly.
Iron Deficiency Without Anemia
Iron deficiency without anemia is a condition where the body has insufficient iron levels, even though the red blood cell count and hemoglobin levels remain normal. The first warning sign that the body is starting to experience iron deficiency is a reduction in serum ferritin levels (SF < 10 μg/L in women, SF < 15 μg/L in men), indicating that iron stores are beginning to deplete.
Although iron deficiency without anemia does not yet lead to a reduction in hemoglobin levels, it can affect overall well-being. Symptoms may include chronic fatigue, weakness, difficulty concentrating, pale skin, feeling cold, and sleep disturbances. If the deficiency worsens, iron deficiency anemia may develop.
Anemia (Iron Deficiency Anemia)
Anemia, also known as iron deficiency anemia, is a condition in which the number of red blood cells or hemoglobin levels fall below normal. Iron deficiency is one of the most common causes of anemia, alongside vitamin B12 deficiency, folic acid deficiency, and anemia associated with chronic diseases. A key indicator of iron deficiency anemia is a decrease in mean corpuscular volume (MCV < 82 fl). When combined with reduced hemoglobin levels (Hb < 12 g/dL in women, Hb < 13 g/dL in men), hematocrit (Ht < 37% in women, Ht < 40% in men), and red blood cell count (RBC < 3.5 × 10^6/µL in women, RBC < 4.2 × 10^6/µL in men), this points to microcytic anemia, which is typical of iron deficiency.
Clinical symptoms of iron deficiency, which can lead to anemia, include chronic fatigue, weakness, rapid heartbeat (especially during physical exertion), headaches and dizziness, reduced immunity, pale skin, dryness, cracks at the corners of the mouth, menstrual irregularities, sleep disturbances, and weakened hair and nails.
If you notice these symptoms, it’s important to consult a doctor and undergo the appropriate tests (see Chapter 6). Untreated clinical iron deficiency can lead to serious health consequences, including impaired oxygen transport, affecting the functioning of the entire body. Remember that iron deficiency can result not only from dietary factors but also from issues with iron absorption in the gastrointestinal tract, such as celiac disease, bariatric surgeries, or bacterial infections (e.g., Helicobacter pylori).
Iron Deficiency in Children
Iron deficiency in children can significantly impact their growth, development, and daily functioning. Common symptoms include:
- Fatigue and Irritability: Children may become lethargic and easily frustrated, affecting their activity levels and willingness to play.
- Pale Skin: Particularly noticeable on the face and the inner eyelids, caused by insufficient oxygen supply to tissues.
- Concentration and Learning Difficulties: Iron deficiency can lead to learning challenges, memory problems, and reduced concentration.
- Developmental Delays: Growth may slow, and developmental milestones might be delayed, impacting the child’s physical and mental development.
- Reduced Immunity: Increases susceptibility to infections and illnesses, often resulting in frequent school absences.
Early detection and treatment of iron deficiency are crucial. Regular blood tests, a balanced diet rich in iron, and appropriate supplementation can prevent the negative effects of deficiency. If iron deficiency is suspected in a child, consulting an experienced clinical dietitian or physician is advisable to ensure proper treatment and support.
Iron Deficiency in Adolescents
During adolescence, the need for iron increases, especially for girls due to menstruation and for boys who experience muscle mass growth. Iron deficiency in teenagers can lead to decreased physical performance and the symptoms mentioned earlier. The heightened needs of the body during this developmental period make monitoring iron levels and maintaining an appropriate diet crucial to avoid more serious health issues.
Symptoms and Consequences of Iron Deficiency in Pregnant Women
Iron deficiency is a common issue among pregnant women, affecting one in two expectant mothers, with anemia occurring in over 25% of cases [12]! This condition usually manifests as fatigue, pale skin, dizziness, shortness of breath, and general weakness. The demand for iron increases during pregnancy due to several key factors:
- Increased blood volume: The expansion of blood volume supports the growing fetus and placenta, raising the need for iron to produce hemoglobin.
- Fetal development: Adequate iron levels are essential for the proper development of the baby’s circulatory system, as the fetus builds its own iron stores.
- Preparation for childbirth: In the third trimester, a woman’s body prepares for potential blood loss during delivery, further increasing iron requirements.
Iron in Pregnancy – Risks and Consequences of Deficiency
Iron deficiency in pregnant women can result in adverse outcomes, including preterm birth, low birth weight, and impaired neurodevelopment of the fetus. For the mother, anemia elevates the risk of complications during childbirth, such as postpartum hemorrhage. Therefore, monitoring iron levels and ensuring adequate dietary intake during pregnancy are essential for maternal and fetal health.
Figure 6. Iron Deficiency and the Health of Mother and Child. Pregnancy is a particularly demanding time in terms of nutritional needs, with iron playing a crucial role in the health of both the mother and the developing baby. Iron affects the body in many ways, including supporting oxygen transport to cells and fetal development. Iron deficiency in pregnant women can lead to serious complications, such as anemia, which can, in turn, affect the proper development of the baby. Therefore, addressing iron deficiency through diet is of utmost importance during this critical period!
Iron Deficiency and Overweight/Obesity
The relationship between iron deficiency and overweight—seemingly unrelated health issues—is quite significant. Individuals with excess body weight often consume processed foods that are high in calories but low in essential nutrients, including iron. Iron deficiency poses an even greater challenge for obese individuals, as excessive body weight is often associated with chronic inflammation, leading to increased production of hepcidin—a hormone that regulates iron levels in the body (see Chapter 5). Elevated hepcidin levels reduce iron absorption in the intestines, exacerbating iron deficiency. Iron deficiency in obese individuals can also complicate effective weight management and worsen health problems related to obesity. If you are overweight or obese and experience symptoms of iron deficiency, such as chronic fatigue, concentration problems, or weakness, it is advisable to consult a clinical dietitian or physician and undergo the necessary diagnostic tests (see Chapter 6).
Iron Deficiency in Athletes
Athletes, particularly those engaged in endurance sports, are especially susceptible to iron deficiency. Among them, three groups are most at risk:
- Female athletes
- Athletes following vegetarian and vegan diets
- Long-distance runners
The increased demand for iron in athletes arises from its critical role in oxygen transport and muscle recovery following intense exercise. Furthermore, rigorous training boosts the production of red blood cells and enhances oxygen metabolism, thereby elevating the body’s iron requirements.
In endurance athletes, increased iron losses occur due to several factors. These include a shortened lifespan of red blood cells, heightened oxidative stress that damages the membranes of red blood cells, and the potential occurrence of microbleeding in the intestines.
Long-distance runners are particularly susceptible to a phenomenon known as foot-strike hemolysis (also referred to as runner’s anemia), which involves the physical destruction of red blood cells in the small capillaries of the feet during repetitive impacts on hard surfaces, such as asphalt or concrete. In runners, the destruction of red blood cells is typically four times higher than in other athletes. Moreover, studies have shown that hemolysis is more pronounced in runners training in shoes with hard soles compared to those using footwear with soft cushioning. This indicates that cushioning is important not only for comfort but also for minimizing iron losses.
Hemoglobin, released from the breakdown of red blood cells, along with the iron it contains, is not always fully recovered by the body, leading to its excess in the bloodstream. In extreme cases, particularly during intense training, the kidneys may become overwhelmed, increasing their permeability. As a result, hemoglobin can be excreted in the urine, leading to additional iron loss—a phenomenon known as hemoglobinuria.
In response to physical exercise, the body produces hepcidin, a hormone responsible for regulating iron metabolism. Hepcidin inhibits the absorption of iron from the intestines and hinders the recovery of iron from the breakdown of red blood cells (see Chapter 4). The highest concentration of this hormone occurs approximately 3-6 hours after exercise, but in long-distance runners (even amateurs), elevated hepcidin levels can persist for up to 24 hours post-exercise. Importantly, it is the duration of the exercise, not its intensity, that has the most significant impact on hepcidin levels.
Iron deficiency in athletes can lead to fatigue, decreased performance, and anemia, making it crucial to monitor iron levels and consider appropriate supplementation (see Chapter 10).
Iron Deficiency and Vegetarian Diets
A vegetarian diet, while offering many health benefits, often contains less heme iron, which is primarily found in animal products. Individuals following a vegetarian diet should pay special attention to plant-based iron sources such as lentils, chickpeas, and whole grains. Combining these foods with vitamin C, found in citrus fruits, can enhance iron absorption. Supplementation with vitamin B12 may also be necessary to prevent additional deficiencies.
Iron Deficiency and Vegan Diets
A vegan diet, which eliminates all animal products, poses additional challenges in ensuring sufficient intake of iron and vitamin B12, potentially leading to iron deficiency. Vegans, relying on plant-based iron sources such as legumes, tofu, nuts, and green leafy vegetables, should be mindful of including vitamin C-rich foods to enhance iron absorption. Due to the lack of natural sources of vitamin B12 in a vegan diet, supplementation of this vitamin is usually essential. In some cases, iron supplementation may also be necessary to effectively manage iron deficiency and avoid potential health consequences. The link between iron deficiency and a vegan diet is also discussed in our article “Vegetarianism in Sports”.
Vegetarianism in Sports

Iron Deficiency in the Elderly
Iron deficiency in the elderly population is a serious issue that can lead to specific health problems, including anemia, weakened immune function, and exacerbation of chronic conditions such as heart failure and chronic obstructive pulmonary disease (COPD). Reduced iron absorption in seniors often occurs due to gastric mucosal atrophy and decreased secretion of hydrochloric acid, which hampers the conversion of non-heme iron into a more absorbable form. Additionally, chronic inflammatory conditions common in older adults can increase hepcidin levels, reducing iron bioavailability by inhibiting its release from macrophages and hepatocytes.
Iron deficiency in this age group can also contribute to muscle weakness, increased risk of falls, and cognitive decline, significantly impacting the quality of life and independence of seniors. Effective management of iron deficiency in older adults requires a multidisciplinary approach that includes diagnosing the complex causes of deficiency, regular monitoring of blood parameters, and support through a well-tailored diet, as well as supplementation or pharmacological treatment when necessary.
Identifying the causes of iron deficiency is crucial for implementing effective dietary therapy and treatment, helping to prevent serious health consequences.
7. What Hinders Iron Absorption (“Leaches Iron”)?
Although the absorption of iron from animal sources is not significantly affected by other dietary components (see Chapter 2), several groups of compounds can hinder the absorption of plant-based (non-heme) iron. These include:
- Phenolic Compounds (Tannins): Tannins, found mainly in coffee and tea, are phenolic compounds with strong binding properties. They form insoluble complexes with iron, reducing its absorption in the intestines. To minimize their negative impact, it is recommended to consume coffee and tea at least one hour after meals rich in iron.
- Phytates: Phytic acid and its salts (phytates) are found in whole grain products, nuts, and legumes. Like tannins, phytates form insoluble complexes with iron, limiting its absorption. People on plant-based diets should be particularly cautious. Thermal processing (cooking, baking) can reduce phytate content but may also lead to a loss of vitamin C, which is crucial for iron absorption. Therefore, it is beneficial to pair phytate-rich foods with vitamin C sources.
- Calcium Salts: Calcium, present in milk, dairy products, and highly mineralized water, can reduce iron absorption since both minerals are absorbed in similar areas and may compete for the same transport mechanisms. It is advisable to avoid combining iron-rich foods with dairy and highly mineralized water during meals.
- Phosphorus Salts: Phosphorus compounds, such as phosphates, can hinder iron absorption by forming poorly soluble salts, such as iron phosphate, which reduce iron’s bioavailability. Products high in phosphorus, like Coca-Cola, may lower the efficiency of non-heme iron absorption when consumed regularly.
- Oxalates: Oxalic acid and oxalates found in vegetables like spinach and rhubarb can negatively impact the absorption of plant-based iron by forming insoluble complexes with iron. Oxalic acid binds with iron, creating complexes that are difficult to absorb. To minimize this effect, consume oxalate-rich foods, such as sorrel, spinach, Swiss chard, and rhubarb, in moderate amounts and separate from iron-rich meals.
- Mineral Antagonism (Zinc, Manganese): Zinc and manganese can compete with iron for the same transport mechanisms in the intestines, potentially reducing iron absorption. Although all these minerals are essential for health, be mindful of their combination in your diet. If you take supplements, avoid consuming zinc and manganese (e.g., multivitamin supplements) alongside iron-rich foods to prevent limiting iron absorption. For the optimal distribution of these supplements, it’s best to consult with an experienced dietitian or physician.
Figure 7. Iron Deficiency and the Vegetarian Diet. For individuals following a plant-based diet, enhancing the bioavailability of non-heme iron, their sole source of this mineral, is crucial. Vitamin C (ascorbic acid), found in citrus fruits and kiwi, significantly improves iron absorption by converting trivalent iron (Fe³⁺) into the more absorbable divalent form (Fe²⁺).
8. Iron Absorption Enhancers
By composing your meals appropriately, you can significantly enhance iron absorption. It’s important to note that certain food components can increase the absorption of this mineral, which is crucial for maintaining its optimal level in the body. Here’s how to effectively support iron absorption through the right selection of food products.
Iron and Vitamin C
Vitamin C (ascorbic acid) significantly enhances the absorption of non-heme iron. It acts as a reducing agent, converting non-heme iron from the trivalent form (Fe³⁺), which is poorly absorbed, to the divalent form (Fe²⁺), which is much better absorbed in the small intestine. It is beneficial to combine sources of vitamin C with iron-rich foods, as this can increase iron bioavailability severalfold! [3]
Which Foods Have the Most Vitamin C?
The highest amounts of ascorbic acid can be found in:
- Citrus fruits (lemons, oranges, grapefruits)
- Kiwi
- Black currants
- Vegetables: red bell pepper, parsley, broccoli, cauliflower, Brussels sprouts
- Fermented foods: sauerkraut, sourdough bread
Keep in mind that vitamin C is sensitive to high temperatures, so it’s best to consume foods containing it in their raw form (e.g., in salads or slaws) or minimally processed.
Acidic Environment (Low pH of Stomach Acid)
An acidic environment in the stomach promotes better iron absorption. Examples of products that lower pH and can enhance iron absorption include:
- citrus fruits (lemons, oranges, grapefruits),
- apple cider vinegar, and
- fermented foods (such as sauerkraut and pickles).
For the same reason, you should avoid consuming alkaline drinks and foods like milk directly before or after iron-rich meals.
Presence of Amino Acids
Amino acids, such as histidine and lysine, found in meat, poultry, fish, and human milk, support iron absorption. Including protein sources in meals enhances the efficiency of iron uptake.
Presence of Fructose
Fructose, found in fruits, also supports iron absorption. It is beneficial to include fruits rich in fructose, such as apples, pears, melons, and mangoes, in your diet to enhance iron absorption.
Figure 8. Supplementation, Iron Deficiency, and Health. Numerous studies indicate that improper treatment of iron deficiency in adults, such as overly frequent intake of iron supplements, can actually reduce its absorption from the gastrointestinal tract. This effect is primarily due to increased levels of hepcidin. Therefore, addressing iron deficiency should ideally be preceded by consultation with a dietitian or physician.
9. Iron Supplementation
Can Iron Tablets Replace Dietary Iron?
Although dietary supplements can effectively address iron deficiency, they should not be used as substitutes for food, and certainly not for preventive purposes. The key principle should be “food first”—prioritize diet. Before opting for supplements, focus on enriching your meals with iron-rich foods (see Chapter 3). A well-balanced diet, rich in this mineral, will usually restore adequate iron levels in the body while providing many other valuable nutrients.
Is Iron Supplementation Worth It?
In cases of diagnosed iron deficiency, when a diet rich in this mineral proves insufficient, supplementation may become necessary. Due to the need to determine appropriate iron dosages, the risk of iron overload, and the potential for side effects, iron supplements should be used only after consultation with a dietitian or doctor.
How to Use Iron Supplements?
Numerous studies indicate that frequent administration of iron supplements can impair its absorption from the gastrointestinal tract. The increase in hepcidin levels stimulated by the first dose inhibits the absorption of subsequent doses by 35–45% for at least 24 hours. This adverse effect intensifies with higher doses. [19, 21].
Guidelines for Taking Oral Iron Supplements:
- Taking on an Empty Stomach: Iron supplements should be taken on an empty stomach or at least two hours after a meal, accompanied only by water or fruit juice (preferably citrus), as most other beverages significantly reduce iron absorption.
- Avoiding Certain Foods: Avoid consuming foods high in fiber, dairy products, and those containing phytates, phosphates, or calcium around the time of supplementation.
- Interactions with Medications: Iron supplements should not be taken with certain medications, particularly proton pump inhibitors, antacids, and some antibiotics (fluoroquinolones, tetracyclines), as these significantly decrease iron absorption.
- Competition with Other Minerals: Avoid taking supplements containing zinc and manganese simultaneously with iron, as they compete for the same absorption sites.
- Morning or Evening Supplementation: Iron is best taken when hepcidin levels are lowest, such as in the evening. Due to high hepcidin levels during physical activity, it is advisable to schedule iron supplementation away from workout times.
Forms of Iron in Supplements
According to European Union regulations [27], 17 iron compounds can be used in dietary supplements. Below are some of the most popular:
Inorganic Iron (Ferrous, Fe²⁺):
- Ferrous Sulfate (FeSO₄): The most commonly used iron salt in supplements, considered the standard. It is well absorbed but may cause side effects like stomach pain or constipation.
- Ferrous Gluconate (C₁₂H₂₂FeO₁₄): An alternative form with similar bioavailability, potentially causing fewer side effects.
- Ferrous Fumarate (C₂H₂(COO)₂Fe): Similar properties as the above.
Inorganic Iron (Ferric, Fe³⁺):
- Ferric Pyrophosphate (Fe₄O₂₁P₆): Available as sucrosomal iron, where ferric pyrophosphate molecules are protected by a double phospholipid membrane patented as Sucrosome®. This form is designed for better bioavailability and excellent tolerance [22].
Organic Iron (Ferrous, Fe²⁺):
- Ferrous Bisglycinate (C₄H₁₀FeN₂O₄): A chelate of iron with glycine, classified as organic iron, where the central iron atom (Fe²⁺) is bound to ligands, forming a stable complex. It is more easily absorbed than inorganic iron salts and is well tolerated.
Elemental Iron:
- Elemental Iron: Iron in its free state that ionizes in the gastrointestinal tract. It has high bioavailability and causes fewer side effects compared to soluble iron salts.
Iron supplements are often combined with vitamin C and folic acid. There are also sustained-release (SR) formulations of iron, which can be suitable for individuals who do not tolerate immediate-release oral preparations. Additionally, products containing heme iron, such as powdered hemoglobin, are available on the market.
In cases of overdose, iron can have toxic effects on the body, which is why the maximum amount of iron allowed in the recommended daily dose of dietary supplements in Poland is set at:
- 20 mg in supplements intended for the general population
- 30 mg in supplements labeled as dedicated for pregnant women [28]
Side Effects
The most common side effects associated with oral iron supplementation affect the gastrointestinal tract and include nausea, abdominal pain, constipation, and diarrhea. Dark stool coloration due to the presence of unabsorbed iron is a natural occurrence and is not harmful to health.
10. Symptoms of Iron Excess: Hemochromatosis
Excess iron, although much rarer than deficiency, poses a serious health risk. High levels of iron in the body can lead to significant health problems, which are equally, if not more, concerning than the consequences of iron deficiency. Hemochromatosis, a condition characterized by chronic iron accumulation, is one of the most severe diseases caused by iron overload.
What Are the Risks of Excess Iron in the Diet?
Excess iron in the body is toxic. This condition catalyzes the formation of free radicals, which damage cell membrane lipids and organelles, leading to cell death. High levels of iron enhance collagen synthesis, which can lead to organ fibrosis. In extreme cases, iron deposits accumulate in the liver, pancreas, heart, and gonads, potentially causing permanent damage to these organs. Symptoms of iron overload include abdominal pain, weakness, fatigue, heart rhythm disturbances, darkening of the skin, and joint pain. Prolonged excess iron can lead to cirrhosis of the liver, diabetes, heart failure, and increase the risk of cancers, particularly liver cancer.
Those at risk of iron overload include individuals engaging in prolonged, uncontrolled supplementation, often motivated by the belief in the ergogenic effects of iron, such as athletes involved in endurance sports like cycling or running. High ferritin levels, indicative of iron overload, are frequently observed in this group. Additionally, individuals with genetic predispositions to hemochromatosis, inheriting mutations in the HFE genes (e.g., C282Y, H63D), are particularly vulnerable to iron excess and should regularly monitor their iron levels.
11. Summary
Iron plays many crucial roles in the body, from oxygen transport and supporting energy metabolism to influencing gene transcription and strengthening the immune system. Therefore, regular blood tests, at least once a year, are essential to detect any potential deficiencies in time. A lack of iron can lead to serious health issues that are better prevented than treated. A balanced diet rich in natural sources of this mineral can help maintain optimal levels.
To address iron deficiency, it is advisable to start by increasing the intake of iron-rich foods, both heme (animal-based) and non-heme (plant-based). In many cases, this approach is sufficient to restore balance in the body without the need for supplements.
Excess iron can be far more dangerous than its deficiency, which is why the decision to supplement should be carefully considered and preceded by a consultation with a dietitian or doctor.
At Made By Diet®, we have extensive experience working with individuals struggling with iron metabolism issues. If you need support in creating a diet that provides the right amount of iron or are dealing with anemia or low iron absorption, we are here to help. Together, we will find the best solution for you!
12. References
- Badania laboratoryjne w dietetyce, A. Dittfeld, D. Parol, Patrycja Pieszczek-Bober, Natalia Mogiłko, Edra Urban & Partner, Wrocław 2024 https://pzwl.pl/Badania-laboratoryjne-w-dietetyce,252927540,p.html
- Współczesna dietoterapia, Lange Ewa, Włodarek Dariusz, PZWL, Warszawa 2022 https://pzwl.pl/Wspolczesna-dietoterapia,197971021,p.html
- Piskin E, Cianciosi D, Gulec S, Tomas M, Capanoglu E. Iron Absorption: Factors, Limitations, and Improvement Methods. ACS Omega. 2022 Jun 10;7(24):20441-20456. doi: 10.1021/acsomega.2c01833. PMID: 35755397; PMCID: PMC9219084. https://pubmed.ncbi.nlm.nih.gov/35755397/
- Wegetarianizm w sporcie, artykuł na stronie Made By Diet: https://madebydiet.com/wegetarianizm-w-sporcie/
- Iron Deficiency Best Practice Guidelines, Australian Institute of Sport, August 2022 https://www.ais.gov.au/__data/assets/pdf_file/0020/1066133/Iron-supplement-best-practice-guidelines.pdf
- Niedobór żelaza u dorosłych – rozpoznawanie i leczenie, Joanna Matuszkiewicz-Rowińska, Jolanta Małyszko, TERAPIA, 1/2023 https://journals.indexcopernicus.com/api/file/viewByFileId/2017574
- Samek, Gracja, and Patrycja Gogga, Zapobieganie niedoborowi żelaza u osób stosujących dietę wegańską, Medycyna Ogólna i Nauki o Zdrowiu 28.1 (2022): 33-39.https://doi.org/10.26444/monz/143826
- Bell S, Rigas AS, Magnusson MK, Ferkingstad E, Allara E, Bjornsdottir G, Ramond A, Sørensen E, Halldorsson GH, Paul DS, Burgdorf KS, Eggertsson HP, Howson JMM, Thørner LW, Kristmundsdottir S, Astle WJ, Erikstrup C, Sigurdsson JK, Vuckovic D, Dinh KM, Tragante V, Surendran P, Pedersen OB, Vidarsson B, Jiang T, Paarup HM, Onundarson PT, Akbari P, Nielsen KR, Lund SH, Juliusson K, Magnusson MI, Frigge ML, Oddsson A, Olafsson I, Kaptoge S, Hjalgrim H, Runarsson G, Wood AM, Jonsdottir I, Hansen TF, Sigurdardottir O, Stefansson H, Rye D; DBDS Genomic Consortium; Peters JE, Westergaard D, Holm H, Soranzo N, Banasik K, Thorleifsson G, Ouwehand WH, Thorsteinsdottir U, Roberts DJ, Sulem P, Butterworth AS, Gudbjartsson DF, Danesh J, Brunak S, Di Angelantonio E, Ullum H, Stefansson K. A genome-wide meta-analysis yields 46 new loci associating with biomarkers of iron homeostasis. Commun Biol. 2021 Feb 3;4(1):156. doi: 10.1038/s42003-020-01575-z. PMID: 33536631; PMCID: PMC7859200. https://pubmed.ncbi.nlm.nih.gov/33536631/
- Kompendium Farmakologii, redakcja naukowa Waldemar Janiec, Wydawnictwo PZWL, Warszawa 2021 https://pzwl.pl/Kompendium-farmakologii,155343967,p.html
- Rejestr Produktów Leczniczych. Charakterystyka Produktu Leczniczego Sorbifer Durules, 100 mg Fe(II) + 60 mg, tabletki o przedłużonym uwalnianiu https://rejestry.ezdrowie.gov.pl/rpl/search/public
- Abioye AI, Okuneye TA, Odesanya AO, Adisa O, Abioye AI, Soipe AI, Ismail KA, Yang JF, Fasehun LK, Omotayo MO. Calcium Intake and Iron Status in Human Studies: A Systematic Review and Dose-Response Meta-Analysis of Randomized Trials and Crossover Studies. J Nutr. 2021 May 11;151(5):1084-1101. doi: 10.1093/jn/nxaa437. PMID: 33758936. https://pubmed.ncbi.nlm.nih.gov/33758936/
- Normy żywieniowe dla populacji Polski i ich zastosowanie. Rychlik Ewa, Stoś Katarzyna, Woźniak Agnieszka, Mojska Hanna, Narodowy Instytut Zdrowia Publicznego – Państwowy Zakład Higieny, Warszawa 2024. https://ncez.pzh.gov.pl/wp-content/uploads/2024/12/normy-31_12.pdf
- Fizjologia Człowieka – zintegrowane podejście, Dee Unglaub Silverthorn, Wydawnictwo PZWL, Warszawa 2019 https://pzwl.pl/Fizjologia-czlowieka.-Zintegrowane-podejscie,75276299,p.html
- Means Jr RT, ed. Nutritional Anemia. In: Nutritional Anemia: Scientific Principles, Clinical Practice, and Public Health. Cambridge University Press; 2019 https://doi.org/10.1017/9781139023993
- B. Frączek, J. Krzywański, H. Krzysztofiak, Dietetyka sportowa , PZWL Wydawnictwo Lekarskie, Warszawa, 2019 https://pzwl.pl/Dietetyka-sportowa,98649389,p.html
- Haematology and Immunology , Philip Xiu & Shreelata Datta, Elsevier Limited, 2019 https://shop.elsevier.com/books/crash-course-haematology-and-immunology/helbert/978-0-7020-7363-2
- A. Bean, Żywienie w sporcie , Zysk i Spółka Wydawnictwo, Poznań 2019 https://pzwl.pl/Zywienie-w-sporcie,94220318,p.html
- Haider LM, Schwingshackl L, Hoffmann G, Ekmekcioglu C. The effect of vegetarian diets on iron status in adults: A systematic review and meta-analysis. Crit Rev Food Sci Nutr. 2018 May 24;58(8):1359-1374. doi: 10.1080/10408398.2016.1259210. Epub 2017 Jul 5. PMID: 27880062. https://pubmed.ncbi.nlm.nih.gov/27880062/
- Stoffel N.U., Cercamondi C.I., Brittenham G. i wsp.: Iron absorption from oral iron supplements given on consecutive versus alternate days and as single morning doses versus twice-daily split dosing in iron-depleted women: two open-label, randomised controlled trials. Lancet Haematol 2017, 4: e524–533. https://pubmed.ncbi.nlm.nih.gov/29032957/
- Niedobór żelaza a dieta wegetariańska? Mamy na to sposób! Blog na stronie Zdrowy nawyk życia, 13 luty 2018 (dostęp 11 sierpnia 2024 r.)
- Moretti D., Goede J.S., Zeder C. i wsp.: Oral iron supplements increase hepcidin and decrease iron absorption from daily or twice-daily doses in iron-depleted young women . Blood 2015, 126: 1981–1989.https://pubmed.ncbi.nlm.nih.gov/26289639/
- Gómez-Ramírez, S.; Brilli, E.; Tarantino, G.; Muñoz, M. Sucrosomial® Iron: A New Generation Iron for Improving Oral Supplementation . Pharmaceuticals 2018 , 11, 97. https://pubmed.ncbi.nlm.nih.gov/30287781/
- Kuras M., Zielińska-Pisklak M., Perz K., Żelazo i cynk – główne mikroelementy niezbędne do prawidłowego funkcjonowania organizmu , Lek w Polsce, VOL 25 NR 05’15 (288). https://www.researchgate.net/publication/280598065_Zelazo_i_cynk-glowne_mikroelementy_niezbedne_do_prawidlowego_funkcjonowania_organizmu
- Dietoterapia , Dominika Głąbska, Lucyna Kozłowska, Ewa Lange, Dariusz Włodarek, PZWL Wydawnictwo Lekarskie, Warszawa 2014 https://pzwl.pl/Dietoterapia,4930593,p.html
- Podstawy żywienia i dietoterapia, Peckenpaugh Nancy J., Wydawnictwo Medyczne Urban & Partner, Warszawa 2012
- Żywienie Człowieka, Podstawy nauki o żywieniu, tom 1 , Redakcja naukowa Jan Gawęcki, Wydawnictwo Naukowe PWN, Warszawa 2012
- Rozporządzenie Komisji (WE) nr 1170/2009 z dnia 30 listopada 2009 r. zmieniające dyrektywę 2002/46/WE Parlamentu Europejskiego i Rady oraz rozporządzenie (WE) nr 1925/2006 Parlamentu Europejskiego i Rady w odniesieniu do wykazów witamin i składników mineralnych oraz ich form chemicznych, które można dodawać do żywności, w tym do suplementów żywnościowych
- Uchwała nr 20/2019 Zespołu do spraw suplementów diety z dnia 13 grudnia 2019 r. w sprawie wyrażenia opinii dotyczącej maksymalnej dawki żelaza w zalecanej dziennej porcji w suplementach diety
- Dawson B, Goodman C, Blee T, Claydon G, Peeling P, Beilby J, Prins A. (2006). Iron supplementation: oral tablets versus intramuscular injection. Int J Sport Nutr Exerc Metab, 16(2), 180-6. https://pubmed.ncbi.nlm.nih.gov/16779924/
- AIS Sports Supplement Framework. Iron, Australian Institute of Sport https://www.ais.gov.au/__data/assets/pdf_file/0014/1000490/Sport-supplement-fact-sheets-Iron-v4.pdf
- Niedokrwistość na tle niedoboru żelaza w diecie , Paweł Lipiński, Rafał R. Starzyński, Agnieszka Styś, Robert Staroń, Anna Gajowiak, Kosmos, Problemy nauk biologicznych, Polskie Towarzystwo Przyrodników im. Kopernika, Tom 63, Numer 3 (304), str. 373-379, 2014
- Kunahowicz H., Nadolna I., Iwanow A., Przygoda B., Wartość odżywcza wybranych produktów spożywczych i typowych potraw , wydawnictwo lekarskie PZWL, Warszawa 2012 https://pzwl.pl/Wartosc-odzywcza-wybranych-produktow-spozywczych-i-typowych-potraw,78924806,p.html
- Rozporządzenie Komisji nr 432/2012 z dnia 16 maja 2012 r. ustanawiające wykaz dopuszczonych oświadczeń zdrowotnych dotyczących żywności, innych niż oświadczenia odnoszące się do zmniejszenia ryzyka choroby oraz rozwoju i zdrowia dzieci Tekst mający znaczenie dla EOG
- Przewodnik do rozporządzenia (WE) nr 1169/2011 w sprawie przekazywania konsumentom informacji na temat żywności, ma na celu pomóc wszystkim podmiotom łańcucha żywieniowego a w szczególności właściwym organom krajowym w lepszym zrozumieniu i prawidłowym stosowaniu rozporządzenia. Niniejszy dokument nie posiada oficjalnego statusu prawnego i w razie sporu ostateczną interpretację prawa dokonują Sądy Administracyjne.
- Niedobór żelaza w krajach rozwijających się, Medycyna Praktyczna, 2004 (dostęp 11 sierpnia 2024 r.)
- Carpenter, C. E., & Mahoney, A. W. (1992). Contributions of heme and nonheme iron to human nutrition. Critical Reviews in Food Science and Nutrition, 31(4), 333–367. https://doi.org/10.1080/10408399209527576
- Andrews SC, Treffry A, Harrison PM. Siderosomal ferritin. The missing link between ferritin and haemosiderin? Biochem J. 1987 Jul 15;245(2):439-46. doi: 10.1042/bj2450439. PMID: 3663170; PMCID: PMC1148141. https://pubmed.ncbi.nlm.nih.gov/3663170/
You may also be interested in:
2025-09-30
Energy Slump After Lunch? How Your Diet at Work Improves Concentration and Productivity
What you put on your plate largely determines how you function at the office. Diet and…
2025-03-20
How Diet and Lifestyle Affect Your Immunity?
The immune system is indispensable to our survival. Its primary function is to constantly…
2025-02-24
The Mediterranean Diet – Discover the Principles and Foods of the Healthiest Cuisine in the World!
This diet influences not only physical health but also the way we think about food and…
 PL (PLN)
PL (PLN) EN (GBP)
EN (GBP) EN (EUR)
EN (EUR)